The active principle of the antifungal and antibacterial drug Situdicine® was lutenurine (also named liutenurin), a mixture of two alkaloids isolated from dried rhizomes of the yellow water-lily (Nuphar luteum (L.) Sibth. and Smith). The two alkaloids were purified under the chlorhydrate forms, and the specific preparation (fast disintegrating tablets) was used to treat skin and mucosal affections, for gynecological applications. The product was effective to treat trichomoniasis in women.
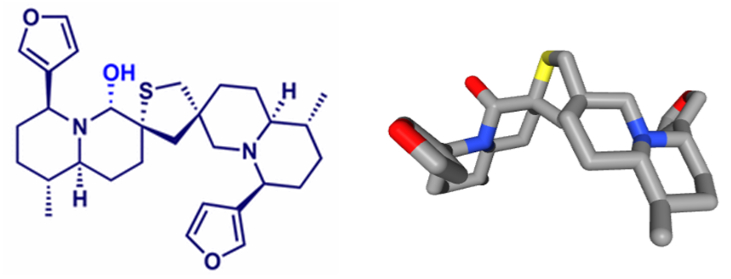
Lutenurine is a mixture of two alkaloids. It may be nuphlein (C30H42N2O4S) and thionupharidine (C30H42N2O2S). But another work referred to 6-hydroxythiobinupharidine and 6’-hydroxythionuphlutine B, two compounds with the same formula (C30H42N2O3S). They are dimeric sesquiterpene thioalkaloids. These furanoquinolizidine alkaloids can be found in the rhizome of different Nuphar plants (nymphaeaceae), notably Nuphar pumilum. They exhibit antibacterial, antifungal and immuno-suppressive effects. Lutenurine inhibited the growth of pathogenic fungi, notably Candida albicans which can be responsible for vulvovaginal candidiasis. It was also a potent antibacterial agent. Situcidine® has been used in the early 1970s for a few years, before being replaced with more active drugs.
Illustration: the female sign (♀) with a flower of yellow water-lily in the center, to evoke the origin of the product and its application in women.