A drug used to treat cough, notably in infants and children. This nonopioid antitussive drug was available under three galenic forms (hence the illustration 1-2-3): an injectable liquid (ampules) used in urgency situations, liquid drops convenient to administer to children, and coated tablets for a common oral usage. The various forms were adapted to treat all types of cough, from children to adults.


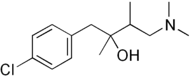
The active principle of Silomat® was clobutinol, a synthetic molecule acting as a cough suppressant, sold as a common over-the-counter (OTC) non-opioid antitussive drug. The drug had first been approved in 1961 and estimated to have had 200 million patient exposures. But later the product was suspected of cardiotoxic effects. It was withdrawn from the US and EU markets (in 2007) due to its potential to induce cardiac arrhythmias by a blockade of the potassium channel coded by the human ether-a-go-go-related gene (hERG). The drug treatment was associated with a risk of introducing torsades de pointes. A clinical study revealed that clobutinol can prolong the QT interval compared to placebo and may thus cause cardiac tachyarrhythmias in some patients. In fact the compound acted as a typical hERG K channel inhibitor, delaying ventricular repolarization and increasing proarrhythmic parameters. Today, the active chemical product (not the drug) remains used occasionally for cough-related research only as a reference product.